Notebooks
Premium
Trends
BioTuring
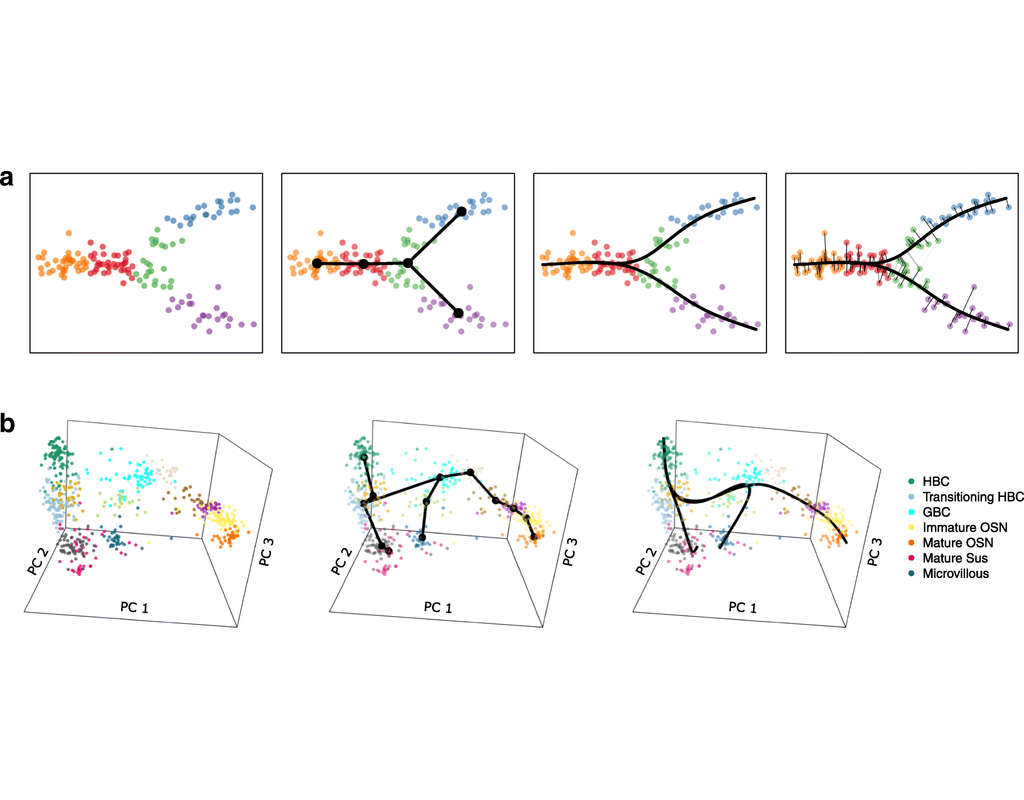
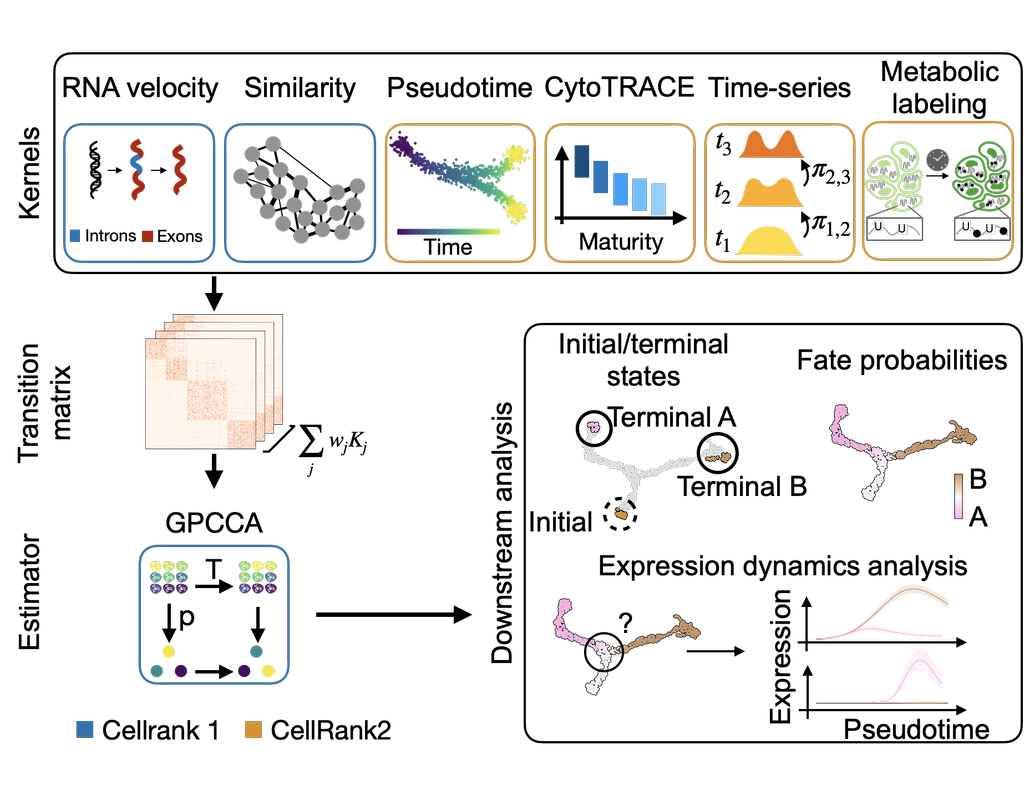
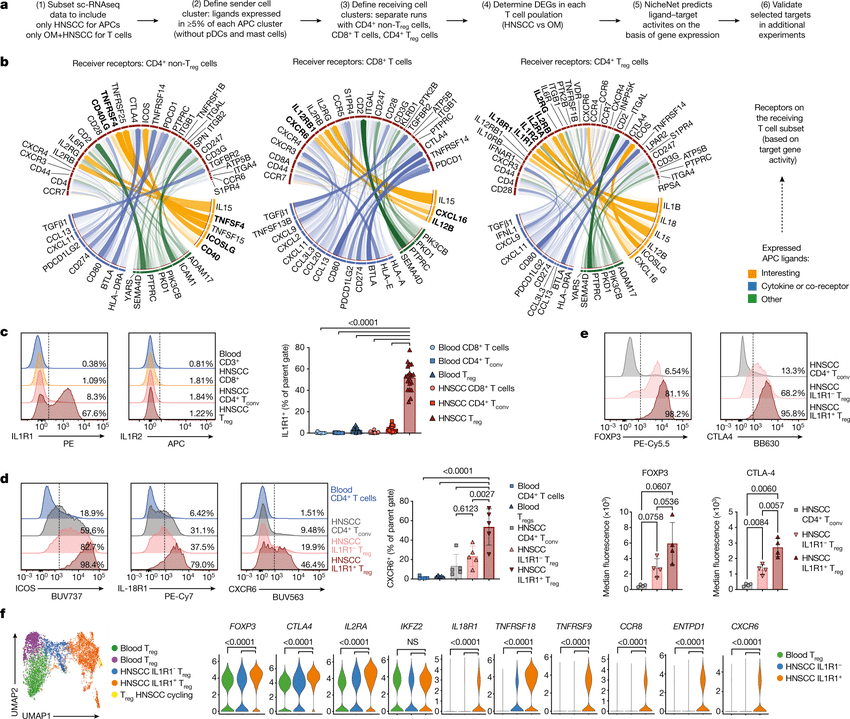
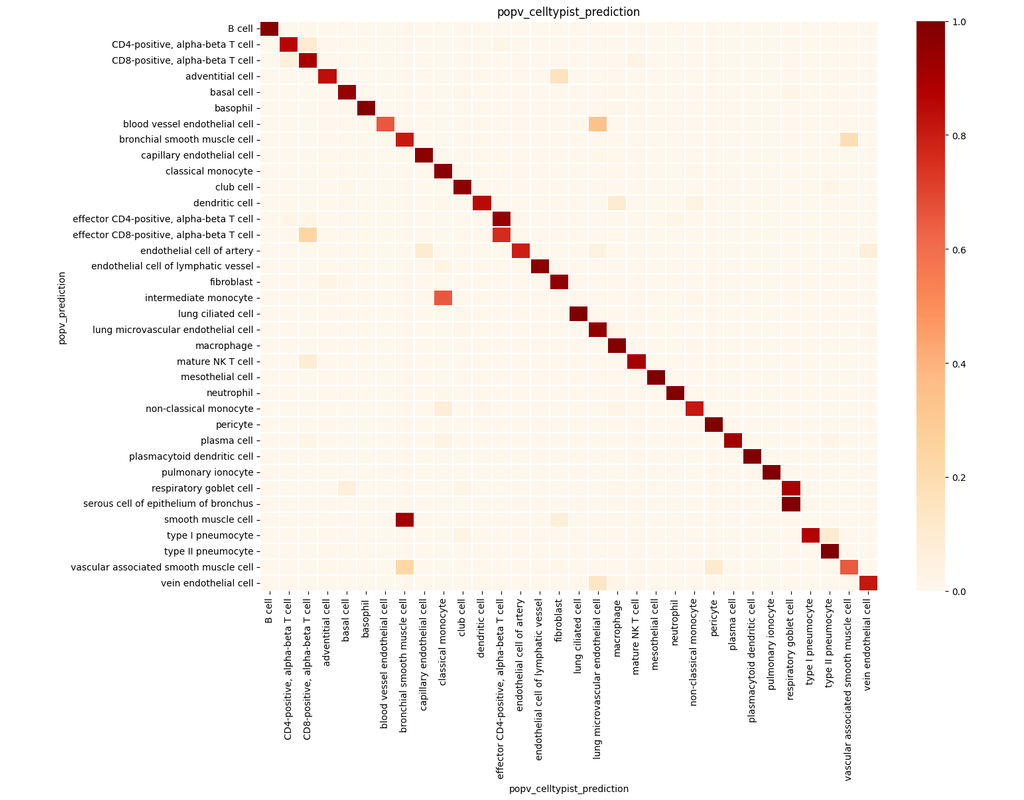
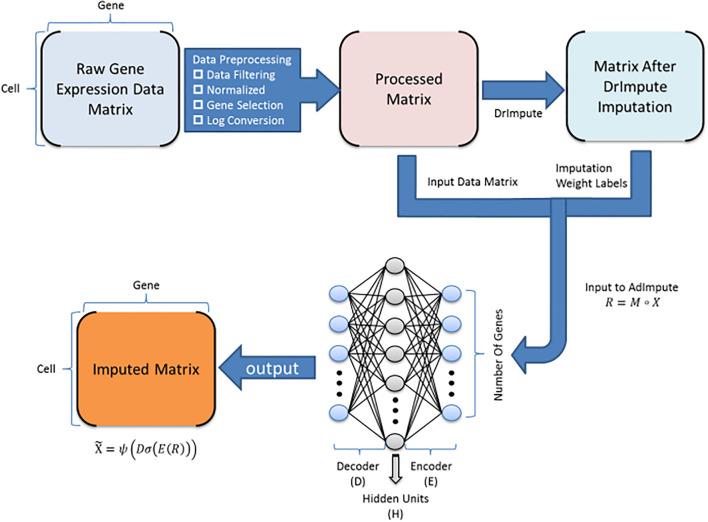
Single-cell RNA sequencing (scRNA-seq) protocols often face challenges in measuring the expression of all genes within a cell due to various factors, such as technical noise, the sensitivity of scRNA-seq techniques, or sample quality. This limitation(More)
| Notebooks |
|---|